Chestnut brown rot management in the field
Results of ongoing research at Michigan State University indicate several available fungicides can significantly reduce brown rot in chestnut.
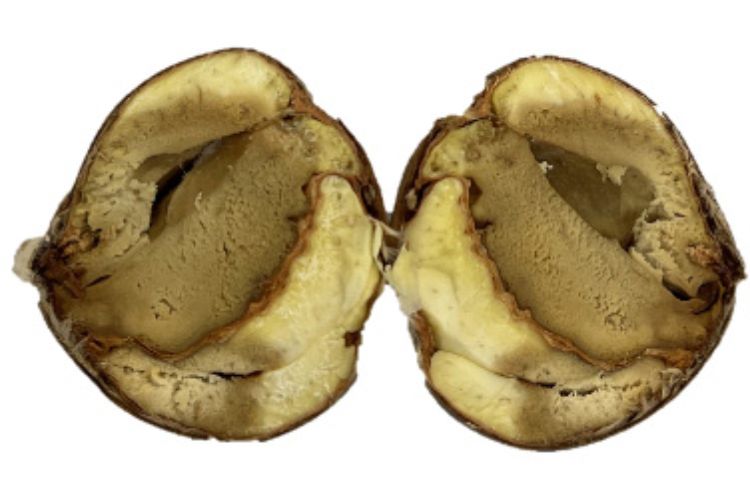
Chestnut rots pose a serious threat to the global production of edible chestnuts (Castanea spp.), significantly affecting their quality and marketability. In Michigan, various pathogens contribute to nut rots, but in recent years brown rot (Photo 1), caused by the fungus Gnomoniopsis smithogilvyi, has become the leading cause of nut decay. G. smithogilvyi was first detected in Michigan in 2016. In a 2017 survey of orchards, 80% tested positive for the pathogen. Since that time, the incidence of brown rot has steadily increased with as much as 9% of the chestnuts processed by the largest cooperative in Michigan showing symptoms of brown rot at harvest. The disease is characterized by a gradual browning of the kernel that develops in the field or after harvest. Recent research at Michigan State University has identified several preventive fungicide treatments that can effectively reduce infection rates and overall disease severity.
As the prevalence of this disease has increased around the world, researchers have been studying its life cycle and disease cycle to understand how and when the pathogen infects chestnuts. There are two types of infections in the disease cycle. The primary infection is caused by sexual spores that overwinter protected by a reproductive structure on dead plant material on the orchard floor. In spring and summer, these sexual spores are released from the understory and create secondary infections that develop asexual spores which are spread by rain splash, wind and insects and cause new infections in a repeating cycle during the growing season. In the fall, cool temperatures again trigger the development of overwintering spores, which then persist into the following growing season and start the cycle all over again.
The spores from the secondary infection cycle can infect flowers, leaves and branches. It is also important to note that the fungus can exist as an endophyte, not causing disease or symptoms—simply surviving on plant parts and debris. However, these endophytic populations become pathogenic when optimal conditions and opportunities for infection occur. When conducive temperature, rainfall and humidity levels coincide with female flower bloom, spores can move to and germinate on female flower parts, entering the plant through the flower and establishing infection in the developing nut embryo.
Fifteen different fungicides were tested in the lab to determine their ability to reduce fungal growth. The fungicides were selected based on research regarding the infection cycle of G. smithogilvyi and related fungi. Under lab conditions, FRAC 3 fungicides (e.g., propiconazole, difenoconazole and mefentrifluconazole) and Bacillus-based products were most effective at suppressing G. smithogilvyi. Seven fungicides were also tested under field conditions with a series of bloom-time applications. The results of these trials indicate that two applications, one at early bloom (Photo 2) followed by a second application two weeks later, are critical to suppressing disease. There is evidence that a third application when the nut is ripening may further decrease disease levels in orchards experiencing high disease pressure from wet/warm weather conditions or historically high infection rates.

Inspire Super (active ingredients difenoconazole and cyprodinil) and Tilt (active ingredient propiconazole) were the most effective at suppressing brown rot in the field trials. Bacillus subtilis fungicides and zinc or potassium phosphonate also significantly reduced disease levels. Despite phosphorus acid products showing lower fungicidal activity in the lab trials, they performed well in the field. This disparity between lab and field efficacy is likely because phosphorus acids work as defense inducers, which improve tree health and resistance to biotic factors and invitro trials were performed on petri plate colonies that don’t have the ability to replicate the chestnut tree’s response to the product. Because of the risk of resistance development, rotate each application between one of the conventional fungicides (Inspire Super or Tilt) and one of the foliar nutrients (potassium or zinc phosphonate).
In addition to fungicide applications, keep trees properly pruned and fertilized to reduce brown rot infection. Orchard floor sanitation, specifically the removal and proper disposal of plant debris (hot compost, bury or burn) is important to limiting overwintering spores and disease pressure in subsequent years. During harvest, nuts should be collected as soon as possible to limit soil contact. Nuts should be transported to the processing plant and cooled as soon as possible to slow the development of any latent infections. Processors should limit the amount of humidity and water utilized during processing and storage to minimize disease development.
Stay connected
For more information on chestnut production, visit the Michigan State University Extension page www.chestnuts.msu.edu and sign up to receive our newsletter with program announcements and timely management information. You can also follow us on Facebook at Michigan State University Chestnut News.
This work is supported by the Midwest Chestnut Producers Council, Michigan Specialty Crop Block Grant Program and many Michigan growers. This work is also supported by the Crop Protection and Pest Management Program [grant no 2024-70006-43569] from the USDA National Institute of Food and Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.



 Print
Print Email
Email