Use of medically important antimicrobials in food-producing animals has declined significantly since implementation of Veterinary Feed Directive guidelines
Based on FDA’s most recent Summary Report on Use of Medically Important Antimicrobials, antimicrobial use in food-producing animals has declined by 28% during the 5-year period since Veterinary Feed Directive Guidelines were enacted in 2017.

Tracking sales of antimicrobials used in food-producing animals
Antimicrobials are a critical tool pork producers use to minimize the impact of disease in their herds. Specific recommendations for managing antimicrobial use are included in the current certification program adopted by pork producers and are described in Pork Industry Guide to Responsible Antibiotic Use. Similar guidelines are available for beef, dairy, poultry, small ruminants, and other livestock industries. To help ensure responsible antibiotic use these guidelines stress the importance of food animal producers working closely with veterinarians to develop disease prevention strategies best matched to their farm, sound record keeping/ transparency, and use of alternatives to antibiotics when possible.
The FDA publishes an “Annual Summary Report for Antimicrobials Sold for Use in Food-Producing Animals” each December. The report describes sales of both medically important (critical medicines used in humans) and medically unimportant (e.g. ionophores and other agents not typically prescribed for human use). Sales are further categorized by species, chemical class, route of administration, domestic vs export use, and whether the compound was sold under a Veterinary Feed Directive (VFD) or over the counter (OTC).
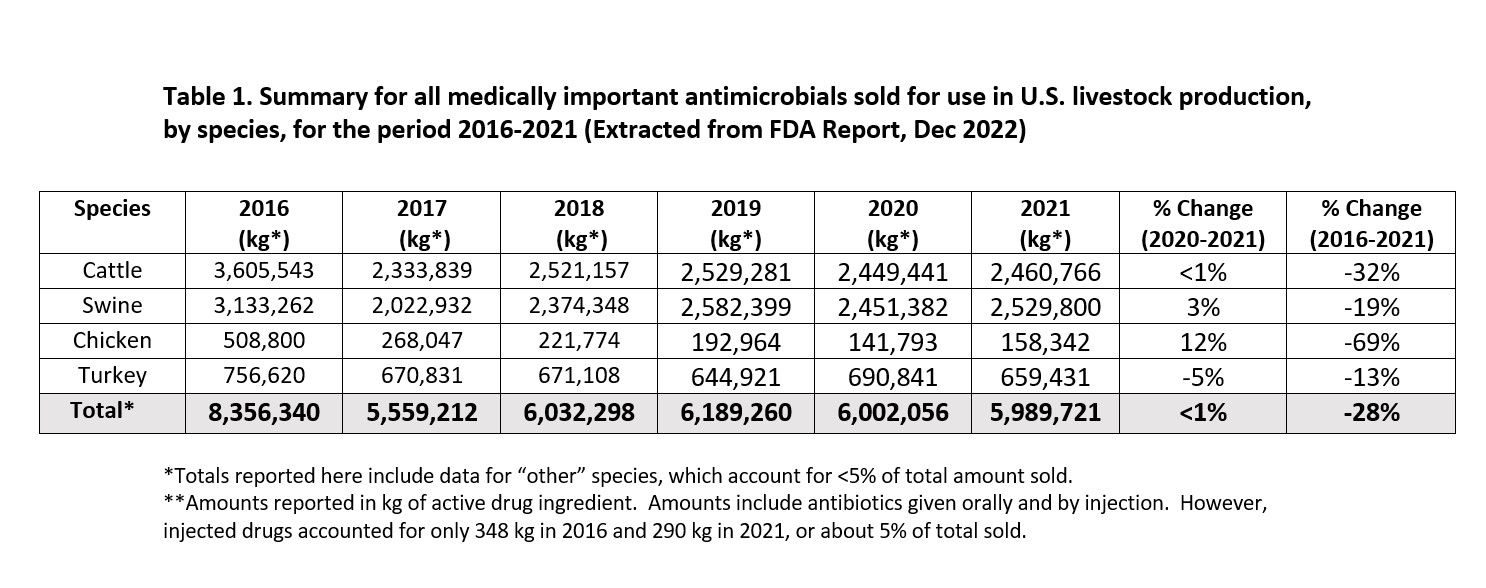 The most recent summary report describes antimicrobial sales during 2021 as it takes almost a year to compile all the data needed for this analysis. This report includes critical data going back to 2009, the first year antimicrobial vendors were required to maintain the database used to compile it. The summary report for 2021 emphasizes changes in sales and use patterns of antimicrobials used in livestock during the 5-year period since VFD was enacted in 2017 and shows that, for all species, their use has declined significantly. When sales data are combined for all medically important antimicrobial sales for all species of livestock, the amount reported as total weight, not number of doses, has declined by 28% since 2106, the last year before VFD. This translates to a net reduction of 2,366,619 kg. of drug as displayed in Table 1, above. Looking at data for sales and distribution of medically important antibiotics for use in pigs only, the amount declined by 19% (from 3,133,262 kg. to 2,529,800 kg.) during the period 2016-2021.
The most recent summary report describes antimicrobial sales during 2021 as it takes almost a year to compile all the data needed for this analysis. This report includes critical data going back to 2009, the first year antimicrobial vendors were required to maintain the database used to compile it. The summary report for 2021 emphasizes changes in sales and use patterns of antimicrobials used in livestock during the 5-year period since VFD was enacted in 2017 and shows that, for all species, their use has declined significantly. When sales data are combined for all medically important antimicrobial sales for all species of livestock, the amount reported as total weight, not number of doses, has declined by 28% since 2106, the last year before VFD. This translates to a net reduction of 2,366,619 kg. of drug as displayed in Table 1, above. Looking at data for sales and distribution of medically important antibiotics for use in pigs only, the amount declined by 19% (from 3,133,262 kg. to 2,529,800 kg.) during the period 2016-2021.
 Closer look at 2021 antimicrobials sold for use in pigs
Closer look at 2021 antimicrobials sold for use in pigs
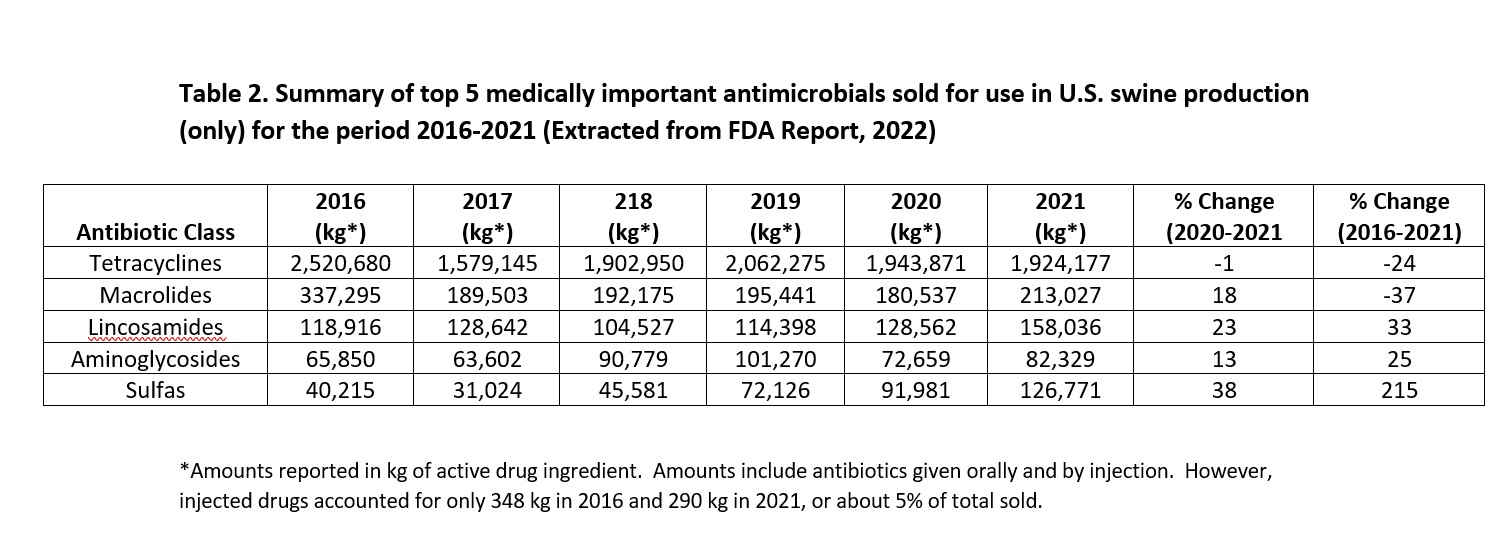
Most sales of medically important antimicrobials used for pork production in 2021 came from sale of tetracyclines as displayed in Table 2. In spite of a 24% (600,000 kg) decline in tetracycline sales volume for pigs since 2016, this class of antimicrobial still accounts for 76% of the total amount used in pigs. This continues a long-standing trend for this class accounting for 75-80% of the total U.S. market for feed grade antimicrobials used in pigs as displayed in Table 2. These compounds possess broad spectrum activity that includes Gram-positive and Gram-negative bacteria, and are frequently used to treat common health issues such as, scours caused by E.coli and respiratory diseases including atrophic rhinitis, and pneumonia caused by Pasteurella and Mycoplasma. Tetracyclines are also inexpensive and easy to administer.
Sales of aminoglycoside and sulfa-containing antimicrobials used in swine continued to increase substantially as displayed in Table 2, with sulfas increasing by 38% in 2021 and by 215% since 2016. Lincosamides increased by 23% in 2021 and 33% since 2016. However, in spite of their recent gains, the overall impact on total antimicrobial sales of these compounds for swine, when combined, was less than 19% of the tetracyclines.
Sales of non-medically important antimicrobials for use in pigs, including ionophores and other small sales volume products not used in human medicine, increased by 61% compared to 2020 and by 44% over 2016 levels. Two important points to consider are that these classes of antimicrobials are not generally used in human medicine, and their use does not require a VFD.
Closer look at 2021 injectable antimicrobials sold for use in all livestock
In the FDA summary report, sales of medically important antimicrobials sold as injectables (e.g., includes some macrolides, aminoglycosides, cephalosporins, quinolones) are combined for all species as displayed in Table 2. Sales of injectables in 2021 declined by 11% from 2016. The combined amount of injectable antibiotics sold for use in livestock in 2021 was only about 5% that of the combined sales of products administered orally.
Possible reasons for the partial rebound in antimicrobial use in pigs since 2017
In 2017, the first year of VFD, sales of medically important antimicrobials for use in pigs declined by greater than 35% from 2016 levels. Although the basis for the approximately 20% rebound since then has not been established, increased herd size and slaughter weight are clearly contributing factors. Slaughter hog number, average weight of hogs at slaughter, and weight of pork produced are key endpoints tracked carefully by the USDA, and these indicators have generally increased by about 2% per year. since 2017. Considering, for example, the weight of pork produced in the U.S. (which increased by 11% from 25 billion pounds in 2016 to 27.7 billion pounds in 2021), it is reasonable to attribute about half the rebound in antimicrobial sales to that factor alone.
A second factor that may have contributed to the increased use of feed-grade antimicrobials in pigs since 2017 is farm labor shortages which have been widely reported. Antimicrobials administered in feed or water, as tetracyclines and sulfa drugs typically are, require less labor than is needed for individual animal administration by injection. Although data on injectables is not broken down by species, the fact that injectables for all species combined declined by 17% between 2016 and 2021, while sales of less labor-intensive in-feed and in-water products increased, is consistent with the possibility that broadly reported shortages in farm labor may have played a role.
An additional factor that may have contributed to the increase purchase of feed-grade antimicrobials is the fear of supply-chain disruption impacts on the availability of antimicrobials. Following the impacts of 2020 and beyond Covid-19 events, farmers have recognized the increased length it takes to get various needed parts, tools, and supplies required for the daily operations of their systems. Shipping delays, labor availability, and manufacturing interruptions have impacted all areas of production in one way or another. When looking to mitigate risks and pre-planning for seasonal health disruptions, having in stock feed-grade antimicrobials available may be the new norm for operations, leading to the 2021 boom in sales, however, this may not speak to actual on-farm usage increases.
Another possible explanation for why sales of certain classes of medically important antimicrobials have rebounded since 2017 is that they were purchased to treat disease conditions that may have increased following VFD implementation. Relevant data on the impacts of VFD on swine health and farm practices across the U.S. will be reported sometime in 2023 from the USDA/NAHMS Swine Survey. However, based on a more limited national survey conducted in 2018 by MSU-Extension titled Veterinary Feed Directive Year 1 in Review, 31% of swine producers who completed the survey reported an increase in swine sickness following implementation of Veterinary Feed Directive (VFD) guidelines, and 18% said they would like to learn more about non-antibiotic options for sick animals. Follow-up action most frequently requested in the survey.
U.S. livestock producers remain committed to responsible use of antimicrobials
Despite the modest increase recorded in 2021 relative to 2020, FDA data suggest overall use of medically important antimicrobials in pigs was still the fourth lowest, for the year, since 2009 when this metric for antimicrobial sales to livestock producers was first reported. Importantly, 98% of medically important antimicrobials sold for use in pigs in 2021 came with a veterinarian’s prescription or under a VFD.
FDA plans to update guidelines for antimicrobial use
The FDA will continue to fine-tune antimicrobial use guidelines to help ensure their responsible use and to preserve the effectiveness of these important medicines for human and animal health. In June 2023 FDA will implement additional steps in this process that will include requiring that all medically important antimicrobials used in food animals be prescribed by a veterinarian. This step will move OTC products under veterinarian oversight and require producers to have in place a valid veterinarian-client-patient relationship (VCPR) with a licensed veterinarian.
Strategies for preventing disease in pigs using management over medication
Strategies to prevent disease in pigs that don’t rely on use of medically important antimicrobials is the subject of a series of articles published by Michigan State University Extension that describe evidence-based strategies that emphasize management over medication. Because pig health issues can vary markedly year-by-year and are often regional or even farm specific, your veterinarian or nutritionist are the best resource to work with on developing strategies for reducing the use of medically important antibiotics in your herd.



 Print
Print Email
Email



