Impacts of Unsafe Food Production
This page corresponds to Training Video 3 in MSU Extension’s 12-video Specialized Meat Processing Variance Training series. Below you will find supplemental information that will help you understand and complete Training Video 3: Impacts of Unsafe Food Production.
Supplmental Information
-
Appendix 4: Bacterial Pathogen Growth and Inactivation
Published on March 18, 2020
This guidance represents the Food and Drug Administration’s (FDA’s) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. -
Bad Bug Book - Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins
Published on March 18, 2020
Food safety is a complex issue that has an impact on all segments of society, from the general public to government, industry, and academia. The Bad Bug Book provides current information about the major known agents that cause foodborne illness. -
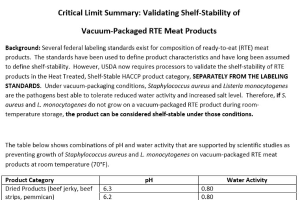
Critical Limit Summary: Validating Shelf‐Stability of Vacuum‐Packaged RTE Meat Products
Published on March 18, 2020
Several federal labeling standards exist for composition of ready‐to-eat (RTE) meat products. The standards have been used to define product characteristics and have long been assumed to define shelf‐stability. -
PROCESSING INSPECTORS' CALCULATIONS HANDBOOK
Published on March 18, 2020
The mission of the Food Safety and Inspection Service (FSIS) is to assure that meat, meat food, poultry, and poultry food products distributed in interstate commerce are wholesome, not adulterated, and properly marked, labeled, and packaged. -
Chapter 3. Factors that Influence Microbial Growth
Published on March 18, 2020
The factors discussed in this section constitute a list of intrinsic, extrinsic, and other factors that may be considered when determining whether a food or category of foods requires time/temperature control during storage to assure consumer protection.
-
416.4 Sanitary operations
Published on March 19, 2020
ll food-contact surfaces, including food-contact surfaces of utensils and equipment, must be cleaned and sanitized as frequently as necessary to prevent the creation of insanitary conditions and the adulteration of product. -
416.11 General rules
Published on March 19, 2020
Each official establishment shall develop, implement, and maintain written standard operating procedures for sanitation (Sanitation SOP’s) in accordance with the requirements of this part. -
416.15 Corrective Actions
Published on March 19, 2020
Each official establishment shall take appropriate corrective action(s) when either the establishment or FSIS determines that the establishment’s Sanitation SOP’s or the procedures specified. -
416.12 Development of Sanitation SOP’s
Published on March 19, 2020
The Sanitation SOP’s shall describe all procedures an official establishment will conduct daily, before and during operations, sufficient to prevent direct contamination or adulteration of product(s). -
416.1 General rules
Published on March 19, 2020
Each official establishment must be operated and maintained in a manner sufficient to prevent the creation of insanitary conditions and to ensure that product is not adulterated. -
416.14 Maintenance of Sanitation SOP’s
Published on March 19, 2020
Each official establishment shall routinely evaluate the effectiveness of the Sanitation SOP’s and the procedures preventing direct contamination of product(s) and shall revise both as necessary to keep them effective with respect to changes. -
416.6 Tagging insanitary equipment, utensils, rooms or compartments
Published on March 19, 2020
When an FSIS program employee finds that any equipment, utensil, room, or compartment at an official establishment is insanitary or that its use could cause the adulteration of product, he will attach to it a ‘‘U.S. Rejected’’ tag. -
416.3 Equipment and utensils
Published on March 19, 2020
Equipment and utensils used for processing or otherwise handling edible product or ingredients must be of such material to facilitate thorough cleaning and to ensure that their use will not cause the adulteration of product during processing. -
416.2 Establishment grounds and facilities
Published on March 19, 2020
The grounds about an establishment must be maintained to prevent conditions that could lead to insanitary conditions, adulteration of product, or interfere with inspection by FSIS program employees. -
416.5 Employee hygiene
Published on March 19, 2020
Cleanliness. All persons working in contact with product, food-contact surfaces, and product-packaging materials must adhere to hygienic practices while on duty to prevent adulteration of product and the creation of insanitary conditions. -
416.17 Agency verification
Published on March 19, 2020
FSIS shall verify the adequacy and effectiveness of the Sanitation SOP’s and the procedures specified therein by determining that they meet the requirements of this part. -
416.13 Implementation of SOP’s
Published on March 19, 2020
Each official establishment shall conduct the pre-operational procedures in the Sanitation SOP’s before the start of operations.
Contact
For customer service or technical support questions when ordering the DVD or online video access, please contact shop.msu.edu customer support.
For questions about the variance, please contact the Michigan Department of Agriculture and Rural Development at 1-800-292-3939. You can also find more information about the meat variance at MDARD’s Meat Processing Regulations and Resources page.
For questions about the meat variance training series and how it can help you, please contact MSU Extension specialist Jeannine Schweihofer at grobbelj@msu.edu.














 Print
Print Email
Email