Oak wilt
The best sampling procedures for accurate oak wilt testing.
Effective oak wilt management starts with confirmation of the disease.
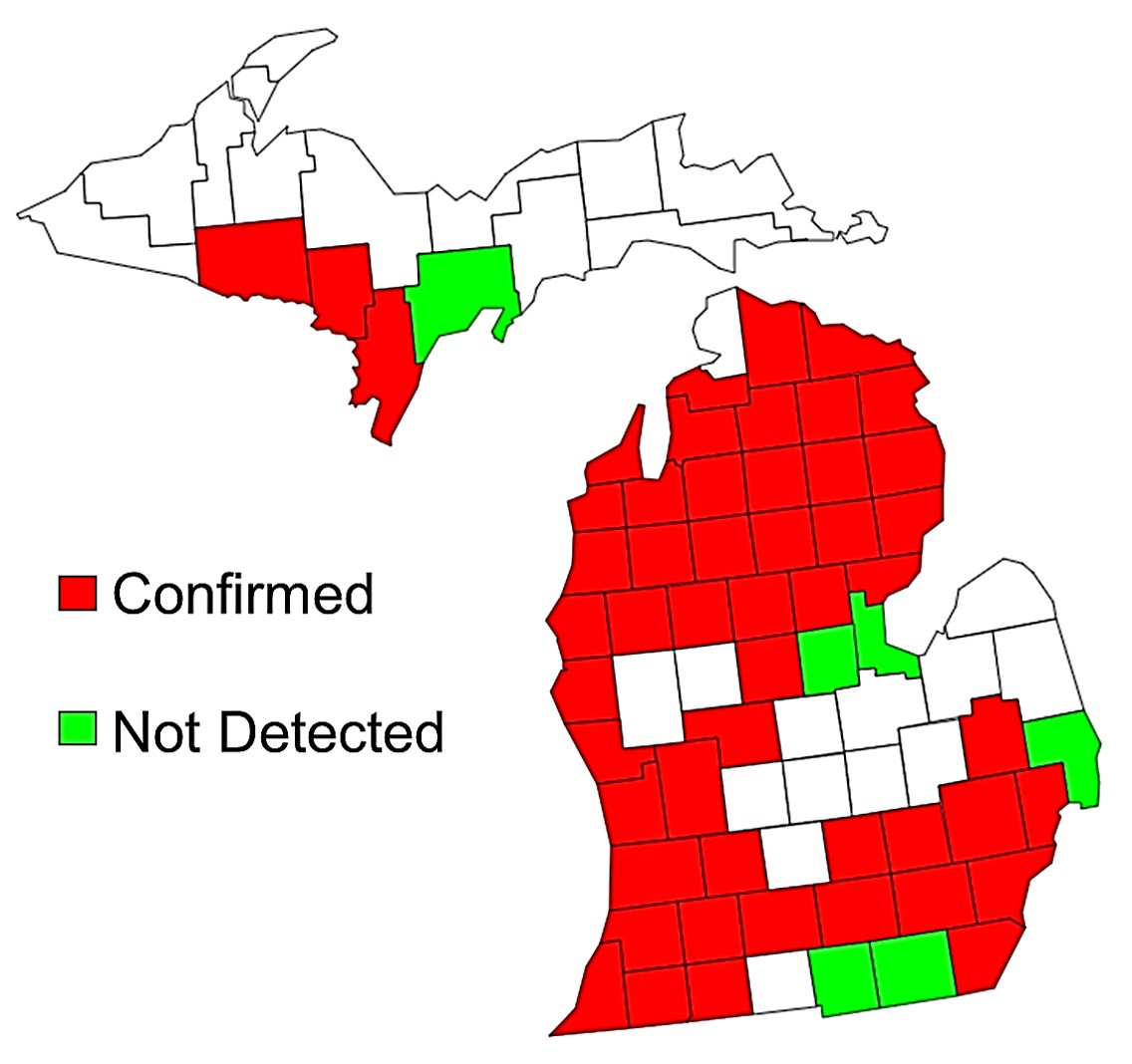
Oak wilt is a fungal disease caused by Bretziella fagacearum (previously known as Ceratocystis fagacearum) and it is widespread in the state of Michigan (see Figure 1). The fungus moves through, and eventually blocks the water conducting system (xylem) in the tree leading to wilting leaves and eventually tree death.
If you suspect oak wilt, here are some pointers on how to collect and submit a good representative sample of the oak tree. Poorly collected samples and/or samples arriving in bad condition cannot be processed for testing because the test will not detect the fungus, even if the tree is in fact infected. This is what we call a false negative result.
To reduce the risk of false negative results, keep in mind these important diagnostic facts:
- The pathogen is found in the outer sapwood of the tree because oak wilt is a vascular disease.
- Recent research has determined that the oak wilt pathogen can also be detected in leaf petioles.
- Sample quality is critical for the success of laboratory testing.
- Effective management of oak wilt depends on accurate disease diagnosis.
MSU Plant and Pest Diagnostics will accept three different types of samples for oak wilt testing, all requiring live tissue:
- Leaf samples. This is a new, non-destructive sampling method recently validated by Dr. Karan Chahal at MSU (see Figure 2).
- Branch samples. This type of sample has been the preferred sampling method for years (see Figures 3-4).
- Bole samples. This highly destructive sampling method creates large wounds in the tree's trunk (see Figure 5).

Leaf sampling guidelines.
Submitting fresh and recently fallen leaves, instead of branch sections or bole samples, is a non-destructive approach that preserves tree integrity while maintaining diagnostic accuracy. This approach has proven effective for disease diagnosis in red oaks, white oaks, and chestnuts during active defoliation in the summer.
- Keep your sampling within 20 feet of the suspect tree, as leaves can be moved by environmental factors.
- Collect 15-20 leaves with attached petioles that exhibit a mix of tan and green on the leaf blade.
- Select leaves that feel fresh and have recently fallen from the tree.
- Include leaves displaying a range of wilt symptoms.
- Symptoms should cover 1-50% of the leaf area.
- Do not collect completely brown or dry leaves.
- Do not remove the petioles from the leaves.
- Place the leaves in a plastic resealable bag to preserve their natural moisture.
- Do not add wet paper towels to the bag, as added moisture promotes mold growth.
- Do not wrap leaves with dry paper, as this will remove their natural moisture.
- Place the samples in a box instead of an envelope.
- Include a completed copy of the General Sample Submission Form.
- Ship the samples using overnight delivery.

Branch sampling guidelines.
Submitting branches for testing is highly recommended, but it can be challenging to achieve when the canopy is too high to reach.
- Whenever possible, select branches with symptomatic leaves. Including some leaves (preferable in a separate bag) along with the branches allow us to better assess the expression of symptoms in the oak tree. There are other diseases and environmental stressors that can resemble oak wilt.
- Ideally, an adequate oak wilt submission should include three (3) sections from different and recently wilted branches that are at least 6 inches long and 1-2 inches thick (see Figure 3). Smaller branch sections are acceptable for testing but pieces of less than half an inch thick will be rejected.
- Remove a small portion of bark and inspect the section of exposed sapwood:
- The sapwood should feel moist to the touch. Dead branch sections are dry and have little weight.
- The inner bark (vascular cambium) should still be green (green arrow in Figure 4).
- The presence of vascular streaking (sapwood discoloration) is desirable as it increases the chance of pathogen detection (see branch on the right side of Figure 4).
- Place oak branches in a plastic bag and keep cold prior to shipping or sample drop-off.
- Do not add wet paper towels or any form of extra moisture to the plastic bag.
- Include a completed copy of the General Sample Submission Form.

Bole sampling guidelines.
Bole sampling is only recommended when the canopy is too high to reach, and the health of the oak tree is already compromised. Taking a sample from the bole (trunk) wounds the oak more severely.
- Remove all the bark from a section of the trunk to expose the sapwood. A hand axe works well for this task.
- Check the sapwood for discoloration. If none is observed, remove the bark from another section of the trunk.
- With a chisel and mallet (or your tools of preference), collect a strip of discolored sapwood (see Figure 5). The strip should be at least 2-3 inches wide and 6-7 inches long.
- Place the strip of sapwood in a plastic bag and keep cold prior to shipping or sample drop-off. Do not send bark pieces to the lab.
- Do not add wet paper towels or any form of extra moisture to the plastic bag.
- Include a completed copy of the General Sample Submission Form.

The Do’s and Don’ts for Testing.
Reliable test results start with sample collection.
|
Do collect live branches or trunk sapwood. Check sapwood for moisture and discoloration. Plant material should feel moist to the touch, not dry. The presence of vascular streaking (sapwood discoloration) increases the chances of pathogen detection. |
Don’t send dry branches or bark pieces. Even if the tree has oak wilt, we cannot detect the pathogen from these types of plant material. Inadequate samples can produce a false negative, which is a negative result when, in fact, the tree has the disease. |
|
Place the tree sample in a plastic bag. This helps retain the naturally occurring moisture in the sample. Also, keep samples cold prior to sending them to the lab. Include some leaves for symptom reference. |
Don’t ship samples on Friday. Samples are not delivered to campus on weekends and may not arrive in the lab in good condition the following week. Remember not to add extra moisture to the plastic bag. |
|
Do fill out a form and place it in a separate bag to keep it clean. Please be sure to include the county where the sample was collected and specify the type of oak (e.g., red oak, white oak, bur oak, etc.). |
Don’t put the form inside the same bag as the plant material. Dirty and wet forms are hard to read. If possible, put the form in a different bag, this will prevent it from getting damp or soiled. |
Inadequate oak samples.
Avoid false negative results.
The following are examples of samples submitted to the lab that do not meet the minimum quality standards for testing. The oak wilt pathogen cannot be detected in these types of samples.
|
Dead and dry pieces of trunk. |
|
|
Pieces of tree bark. |
|
|
The end of a branch (very thin, less than half an inch). Attached leaves do not show disease symptoms. |
Sample fees for oak wilt testing.
Please note, oak samples are charged differently depending on the place of origin.
|
Description |
Fee per sample |
|
In state (Michigan) |
$45.00 |
|
Out of state |
$65.00 |
Additional information.
Please contact MSU Plant & Pest Diagnostics at pestid@msu.edu or 517-355-4536 for questions and further clarification on sample collection and/or shipping.
For more information on oak wilt, read:



 Print
Print Email
Email