Nematodes in commercial vegetables
Fred Warner and George Bird, Nematologists, Michigan State University
Nematodes of Importance in Vegetable Production
Introduction
Nematodes are animals. More specifically, nematodes are non-segmented roundworms, which separate them from their close relatives the segmented roundworms, some known as earthworms. Adult nematodes can vary in length from 1/300 of an inch to nearly 9 feet. Nematodes are commonly found in soil or water, including oceans. They are the most abundant animals on Earth. A shovel-full of garden soil may include as many as a million or more nematodes.

Most species of nematodes are regarded as beneficial. They feed on bacteria, fungi, and other soil-inhabiting or aquatic animals. Some are quite specific in the types of foods they feed upon, others are considered omnivores and potentially feed on a wide range of foods.
Some species of nematodes are parasites of plants and animals. Plant-parasitic nematodes share three common characteristics. First, they are all microscopic, with adults ranging in length from about 1/60th-1/4-inch in length. Secondly, they are obligate parasites of plants, meaning they must have living plant tissue to feed on in order to grow and reproduce. Finally, they all possess stylets, which are structures similar to hypodermic needles these nematodes use to puncture plant cells and to obtain the contents of those cells. All plant-parasitic nematodes spend at least part of their life cycles in soil, although some are found principally in root or leaf tissue.
Every species of plant has at least one species of nematode that parasitizes it. The majority of plant-parasitic nematodes feed on roots (about 95% of the described species) either within the root tissue or outside of it with their bodies in the soil. Some nematodes feed within leaves. Since plant-parasitic nematodes must have living host tissue to feed on to grow and reproduce if the host dies, nematodes will disperse searching for other plants or they will also die.
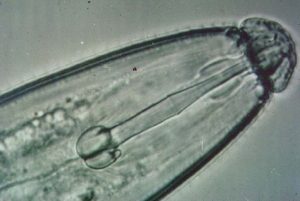
Feeding by plant-parasitic nematodes, in general, does not result in the development of characteristic secondary (aboveground) symptoms. For this reason, nematode problems often go undiagnosed. Typical aboveground symptoms of nematode infections include stunting, yellowing and wilting. In some situations, yields can be significantly reduced. A few types of nematodes do produce characteristic symptoms or signs, but these will be discussed under specific nematodes. Often nematodes will reduce yields without the production of any noticeable above ground symptoms.
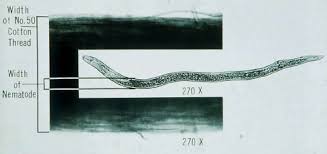
Nematodes are similar to insects in that they possess exoskeletons. This skin must be shed or molted for a nematode to grow. A typical plant-parasitic nematode life cycle consists of an egg, 4 pre-adult stages (referred to as juveniles) and an adult. Females are the most destructive; some males do not feed. There are many species of plant-parasitic nematodes where males are rare or not known to exist. The life cycle of a plant-parasitic nematode may be completed in as little as two weeks or as long as two years depending on the species and the temperature. Nematodes are cold-blooded animals, so temperature plays a crucial role in development. In contrast to plant-parasitic nematodes, some bacterial-feeding nematodes can complete their life cycles in 48-72 hours.
Due to the lack of legs or wings and also due to their microscopic sizes, plant-parasitic nematodes do not move long distances on their own (typical horizontal migration would be less than 6 inches per year). They are usually transported over long distances on machinery, in nursery stock, transplants, seeds, or by animals. Anything that moves soil, moves nematodes including water and wind. Some nematodes, however, are known to move a couple of feet vertically in the soil during a single growing season in response to adverse environmental conditions.
Northern Root-Knot Nematode (Meloidogyne hapla)
Host Plants
Very wide host range including virtually all vegetables. All dicotyledonous plants should be regarded as hosts unless they have been documented by nematologists as non-hosts.
Biology
The northern root-knot nematode (NRKN) overwinters in the soil as eggs in annual cropping systems. As soil temperatures increase in the spring, second-stage juveniles hatch from these eggs, migrate through the soil and penetrate the roots of host plants.
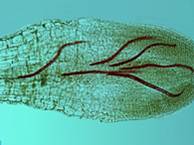
The nematodes establish feeding sites in pro-vascular tissue in an area behind the root cap. As the infected root continues to grow, the vascular tissue differentiates in the area where the nematodes have established their feeding sites so they disrupt the functioning of the phloem and xylem.
Shortly after successfully establishing feeding sites, the second-stage juveniles begin to swell. The nematodes soon molt to third-stage juveniles. Eventually, following two more molts, they mature to become adult female or male nematodes. Females are round and incapable of movement. Males are worm-like and generally exit the root because they do not feed. Female NRKN produce large numbers of eggs, potentially up to 1,000, in a gelatinous matrix secreted by the anus.
The NRKN can complete its life cycle in a month at optimal soil temperatures. Therefore, the nematode can complete multiple generations per growing season.
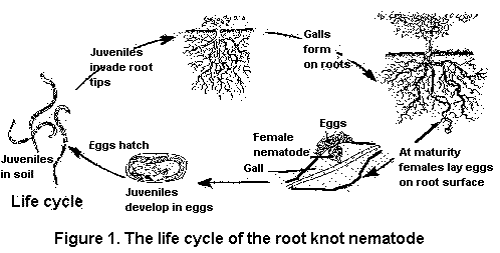
Symptoms
The NRKN, like many other nematode species that feed on vegetables, do not cause characteristic symptoms of the foliage. Typical symptoms are stunting, yellowing and reduced yields. Severely infested plants may wilt during periods of hot, dry weather because the nematodes often negatively impact water transport.
Invasion of roots by NRKN will result in the production of small swellings on roots called galls. Galls will vary in size depending on the numbers of nematodes feeding within them. Carrots and parsnips are highly susceptible to NRKN. Parasitism will result in qualitative losses due to forking or stubbing of the tap roots.


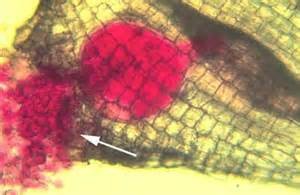
Management
Avoidance: Once established, root-knot nematodes are virtually impossible to eradicate. Therefore, attempts should be made to keep sites free from NRKN for as long as possible. This is primarily accomplished by using nematode-free transplants and by not contaminating fields with NRKN-infested soil.
Population Reduction
Cultural Controls: Sites with histories of root-knot nematode problems should be kept out of vegetable production for a period of 1-3 years. The length of the rotation is primarily dictated by the population density of NRKN recovered and the susceptibility of the vegetable crop. NRKN non-host crops such as corn or small grains should be grown to reduce population densities of these important pathogens. Weed control is imperative as many weeds serve as hosts for the NRKN.
Because root-knot nematodes swell and become immobile after establishing feeding sites, they can be “trapped” within a host. If the host is killed or removed from the soil, NRKN numbers can be reduced. Edible radish can serve as a trap crop but to achieve its full benefit, close monitoring of the nematodes within the roots is necessary. If female nematodes begin to produce eggs, then the benefit of the trap crop is lost. Using radish as a trap crop can be tricky.
Genetic Controls: Some varieties of tomato have a gene (the Mi gene) that infers resistance to the southern root knot nematode, M. incognita, as well as other species of root knots. Some varieties of vegetables may have tolerance to root-knot nematodes but this information is often not readily available.
Chemical Controls: Sites should be routinely sampled for plant-parasitic nematodes prior to planting vegetables especially those extremely susceptible to NRKN. If nematodes are recovered at damage threshold levels, use of a nematicide may be advised.
Cyst Nematodes (Heterodera spp.)

Host Plants
Four species of cyst nematodes occur in Michigan that feed on vegetable crops. However, these species do not exist in all the states that comprise the Midwest. The carrot cyst nematode, Heterodera carotae, has carrot as its only host. The soybean cyst nematode, H. glycines, feeds on green beans and peas. The sugar beet cyst, H. schachtii, feeds on a variety of vegetables in the plant families, Brassicaceae and Cruciferae, primarily including beets, broccoli, cauliflower, cabbage, Brussels sprouts, turnip, and spinach. The final cyst nematode, the clover cyst, H. trifolii, is reported to parasitize some vegetables but is not regarded as a serious pathogen of these crops in Michigan. If we include hop as a vegetable, the hop cyst nematode, H. humuli, has been detected in around the Traverse City area in Michigan. This nematode is currently regarded as the most important nematode parasite of hop.
Biology
Cyst nematodes overwinter as eggs within cysts in the soil. Cysts are the dead remains of females and in the genus, Heterodera, are lemon-shaped and are about the size of a head on a straight pin. In the presence of root exudates, eggs hatch and second-stage juveniles emerge from the cysts, migrate through the soil and penetrate roots. They establish feeding sites just outside the vascular tissue. Similar to root-knot nematodes, they swell as they grow. Eventually, females become large enough that they rupture through the epidermis of the root and are exposed to the soil. Males are worm-like and usually exist the root to mate with females.
Female cyst nematodes produce approximately 50-100 eggs in a gelatinous matrix outside their bodies, but the vast majority of eggs are retained within their bodies. The eggs produced in the matrices typically hatch soon after production whereas; the ones contained within the females may lie dormant for up to 10 years or more. The numbers of eggs produced by cyst nematodes varies by species. Carrot cyst nematodes are much smaller and produce far fewer eggs (100 compared to several hundreds) than the others listed.
Symptoms
Feeding by cyst nematodes usually results in areas of stunted and yellow plants. Yields can be significantly reduced by cyst nematodes. The severity of the symptoms varies according to host and the population densities of the cyst nematodes.
Females can be observed with the naked eye on infected plants during the growing season. Observance of cyst nematode females is a diagnostic sign of infection. Females are white or yellow in color and roughly the size of the period at the end of this sentence. Cyst nematode-infected plants often have reduced root systems. If the host is a legume, root nodulation may also be reduced.
Management
Avoidance
Cyst nematodes can survive in the soil for periods of 10-15 years in the absence of host crops. For this reason, it is best to avoid cyst nematodes for as long as possible. This is usually done by not contaminating fields with cyst nematode-infected soil or, in the case of hop, using hop cyst nematode-infested rhizomes. Growing host plants in long rotations with non-host crops also minimizes the risks of severe cyst nematode problems developing in the future. If cyst nematodes become established in a field, the production goal must change as growers will need to learn how to optimize yields in the presence of these nematodes.
Population Reduction
Cultural Controls: Sites with histories of cyst nematode problems should be kept free from host plants for a period of one or more years. Crop rotation is the most effective tactic to reduce population densities of cyst nematodes. Most of these pathogens have narrow enough host ranges, where at least one or more non-host crops should be economically viable options in any cropping scheme.
Chemical Controls: Cyst nematodes are difficult to control with nematicides. However, use of these materials can result in yield increases and declines in cyst nematode population densities.
Stem Nematode (Ditylenchus dipsaci)
Host Plants
Very wide host range although onion is quite susceptible. Many other vegetables are hosts including beet, carrot, celery, cucumber and tomato. Plants propagated by bulbs, corms and tubers typically are susceptible to stem (also known and stem and bulb) nematodes. This nematode is referred to as the “bloat” nematode on garlic and it is a serious pathogen. Another species in the genus, D. destuctor, the potato rot nematode, can cause serious symptoms on potato but it is unknown in Michigan.
Biology
Stem nematodes overwinter as fourth-stage juveniles and adults. They spend the winter in plant tissue or soil. When moisture is adequate, the nematodes migrate from their overwintering sites onto the leaves and stems of young plants. Females will produce up to 500 eggs and can survive 10 weeks or longer. The life cycle can be completed in about three weeks in optimal conditions. They are active very early in the spring and egg laying can occur at temperatures less than 40 F. Vegetables produced in hot, dry summer conditions are less likely to suffer severe damage from stem nematodes. However, plants infected in the spring may not exhibit symptoms until the summer.
Stem nematodes can persist for long periods of time. They are quite resistant to desiccation and cold temperatures. These nematodes will often form aggregates of large numbers of individuals (sometimes called “eelworm wool”) to survive during adverse conditions. These nematodes become extremely active in water. Therefore, population densities often decline rapidly in the absence of host plants in fields.
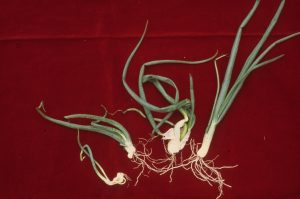
Symptoms
Deformity of leaves and bulbs are the most common symptoms of stem and bulb nematode infestations in onion. Yellowing of leaves may develop with blisters. Young plants are often deformed or may be killed at high infestations if other pathogens are also present. Older, infected bulbs show swelling (bloating) of the scales with cracks often occurring at the root disc of the bulb. These bulbs are often soft and when cut open reveal concentric rings of brown leaf scales. Infected bulbs can rot in storage because they are often invaded with soft-rot bacteria.
Other plants infested with stem nematodes are stunted and distorted. In beet, the growing point is often killed and multiple crowns may develop. Severe crown rot may develop late in the growing season with many vegetables infested with these pathogens.
Garlic bulbs can be infested with bloat nematodes and no symptoms exhibited. Infested bulbs may be more susceptible to gray mold caused be Fusarium.
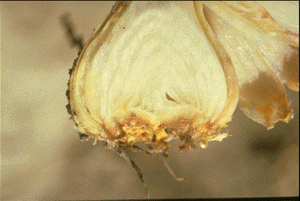

Management
Avoidance
Sites are usually infested by planting stem nematode-infested material. This should be avoided.
Population Reduction
Cultural Controls: Crop rotation or the use of fallow can be employed to reduce population densities of stem nematodes. Some weeds are hosts, so good weed control is essential to ensure success of this tactic. Patterns of wetting and drying of the soil do not favor these nematodes.
Hot water dips can be used for disinfesting sets and bulbs. However, the temperature and time of dipping must be controlled carefully. Often temperatures of 110-115 F are required to kill these nematodes. The time requirement is typically 1-2 hours.
These nematodes typically move on the surfaces of plants in water. Therefore, attempts should be made to minimize leaf wetness.
Chemical Controls: See section on nematicides for information.
Common Needle Nematode (Longidorus elongatus)
Host Plants
Celery, onion and other vegetables serve as hosts for this nematode. Mint is an excellent host.
Biology
Needle nematodes overwinter as juveniles and adults in the soil. Eggs are laid in the spring and early summer when soil temperatures are cool and roots are being produced. Females produce relatively few eggs, typically about 20 per year. Needle nematode adults may live for several years and may require more than a year to complete a generation particularly in temperate climates.
As temperatures rise and soil moisture levels decrease in the summer, needle nematodes migrate deep into the soil. They often rest 2-4 feet below the soil surface. During the fall, they often return to the root zones. Due to this behavior, it is best to sample for these nematodes during the spring or fall.
Symptoms
The most characteristic symptom of feeding by needle nematodes is a swelling of root tips, especially younger roots. Often these tips are killed and many roots emerge behind these areas. Root systems may be seriously stunted.
Needle nematode feeding can result in serious stunting of young plants. The damage is often very evident early in the growing season and stunted plants remain in this condition throughout the year.
Management
Population Reduction
Cultural Controls: Rotation to non-host crops is recommended to reduce population densities of common needle nematodes. Because these nematodes are often present in fairly low numbers and produce few eggs, a year without a host may be sufficient to adequately reduce their numbers.
Chemical Controls: Soil fumigation is effective for control of needle nematodes. However, nonfumigant nematicides are generally not effective.
Pin Nematodes (Paratylenchus hamatus and P. projectus)
Host Plants
Carrot and celery are susceptible to pin nematodes. Anecdotal evidence in Michigan suggests sugar beet can be harmed by pin nematodes so red beet and turnip may also be sensitive. Other vegetables serve as hosts but appear quite tolerant to pin nematodes.
Biology
Pin nematodes typically feed as ectoparasites on roots. Females can produce 2 or 3 eggs per day while feeding. The life cycle can be completed in about 35 days at optimal soil temperatures. Pin nematodes can persist in the soil due to their abilities to produce a quiet pre-adult stage.
Symptoms
Feeding by pin nematodes can result in stunted, unthrifty plants. On carrot, tap roots are stunted and pigmentation is delayed.
Management
Population Reduction
Cultural Controls: Pin nematodes can be managed with rotation although these nematodes do feed on a wide range of plants. Small grains are often used as covers in the fall to minimize soil erosion in fields where vegetables are produced. These cover crops can serve as hosts for pin nematodes. However, pin nematodes are not regarded as serious pathogens of most plants.
Chemical Controls: See section on nematicides.
Stubby-Root Nematodes (Paratrichodorus and Trichodorus spp.)
Host Plants
These two nematode genera have numerous species so there are many hosts. However, in vegetable production, these nematodes are issues in potato due to their abilities to vector the tobacco rattle virus (TRV), one of the causal agents of spraing. Many weeds, particularly common chickweed and shepherd’s purse, serve as hosts and reservoirs for TRV.
Biology
Stubby-root nematodes feed as ectoparasites on roots. Eggs are laid in the vicinity of roots and upon hatching, the young nematodes feed. Stubby-root nematodes can complete multiple generations per growing season and life cycles range from 21-45 days. These nematodes prefer sandy soils that receive moisture. Damage is more severe in sandy locations due to the preferences of these nematodes for soils of this texture.
Symptoms
Feeding by stubby-root nematodes results in stubby root tips hence their common name. Often these roots tips because necrotic (brown/black). If the nematodes transmit TRV to potato, upon slicing the tubers, arcs of discoloration can be observed (spraing).Generally TRV-infected tubers will produce plants with healthy foliage.

Management
Population Reduction
Cultural Controls: These nematodes, like most others, can be managed using rotation to non-host plants. However, stubby-root nematodes often infest fields at low population densities so control may not seem warranted.
Genetic Controls: Potato varieties differ in their susceptibilities to spraing with some reported to have resistance.
Chemical Controls: They can be controlled using fumigant and non-fumigant nematicides.
Sampling Nematode Populations
Plant-parasitic nematodes are microscopic organisms with aggregate distributions in fields. They tend to occur in clumps, therefore, symptoms caused by nematode feeding, occur in circular or elliptical patterns. If aboveground symptoms are uniformly distributed in any given field, the cause of the problem is typically not nematodes.
A great deal of research has gone into sampling nematode populations. A few points of emphasis are below.
- Due to their microscopic nature, the only way to diagnosis a plant-parasitic nematode problem is to collect a soil and/or plant tissue sample(s) and send it to a Nematode Diagnostic Lab for analysis.
- It is impossible to provide specific recommendations for the management of plant-parasitic nematodes unless they are properly identified to genus or species.
- When collecting soil samples for plant-parasitic nematodes, the more soil cores gathered, the better the sample. The collection of roughly 20 soil cores is usually adequate. However, it is only necessary to submit a pint to a quart of soil to a lab.
- Employ different methodologies when sampling in an effort to avoid nematode problems (see 3) than diagnosing them (collect soil and plant tissues in the margins of diseased areas).
- For more complete instructions on sampling for nematodes, please refer to any bulletin or other publication devoted to sampling for these organisms.
Management of Plant-Parasitic Nematodes
The best defense against nematodes is to avoid them. Once fields or plant tissues are infected with nematodes, eradication is usually not a viable option. Nematodes are usually transported over long distances by machinery, in plant material, animals, water or wind. Natural disasters, such as floods, are uncontrollable. However, the patterns in which machinery are moved and the sanitation of this equipment can be controlled. These tactics should be considered when trying to avoid nematodes. The bottom line is anything that moves soil, moves nematodes.
Often, fields do become infested with nematodes. If samples indicate the presence of pathogenic species at damage threshold levels, then steps should be taken to reduce the population densities of these organisms. There are many tactics that can be utilized to accomplish this purpose.
Biological Controls
The majority of nematodes present in the soil are considered beneficial. They typically feed on bacteria, fungi or small animals including other nematodes. Research results indicate, as the abundance of these nematodes increases this often correlates with a decrease in the numbers of plant-parasitic nematodes. Steps can be taken to increase the diversity and numbers of these beneficial nematodes in fields. For information, consult bulletins or other publications focused on soil ecology and health.
Many organisms are parasites or pathogens of nematodes. Most of these occur naturally in soils but often do not provide sufficient enough control of plant-parasitic nematodes to keep their population densities below damage threshold levels. Some products are available that are biological nematicides, but, in general, to date, their uses have not resulted in consistent control of nematodes in Michigan.
Biotechnological Controls
Plants have not been genetically modified at this time against plant-parasitic nematodes.
Chemical Controls
Nematicides are compounds that kill nematodes. Nematicides are classified under two main types, fumigants or nonfumigants. Fumigants are typically compounds sold as liquids that react with water in the soil, to produce gases that kill a wide variety of organisms including plants. They are considered wide spectrum biocides for this purpose. They are usually applied to the soil in the fall (preferably) or spring when soil temperatures are adequate. Fumigant nematicides are labeled for use in vegetable production.
Nonfumigant nematicides are also labeled for use in vegetable production. Unlike fumigants, they do not volatilize in soil water. They can be applied prior to, at or even after planting in some situations. These compounds are not as broad in their spectrum as killing agents. They will control nematodes, but their use will often result in decreases in numbers of beneficial as well as parasitic nematodes.
Cultural Controls
These types of controls include the actual growing of crops. Tactics that impact nematode populations are the species of plants grown, planting dates, presence or absence of companion crops, types of cover crops, etc. Most of these tactics have been covered in detail in the sections where specific nematodes were discussed.
Genetic Controls
Plant resistance tends to be the most sustainable of the control tactics. However, most vegetables do not have resistance against nematodes except many varieties of tomato have resistance to root-knot nematodes. Some varieties of vegetables may tolerate feeding by nematodes more than others, but this information is not always readily available. These data can be obtained with on-farm testing or screening of selected varieties of vegetables by nematologists in controlled experiments.
Physical Controls
These include the use of heat, steam or water (flooding) to reduce population densities of nematodes. In field situations, these types of controls are limited especially in the Midwest. However, in glasshouse or poly-house production of plants, heat or steam is typically used to sterilize growing media.
Conclusions
Vegetables are hosts to many species of plant-parasitic nematodes capable of causing significant yield losses. However, some vegetable crops are much more sensitive than others to certain species of nematodes. For instance, carrots are extremely susceptible to northern root-knot nematodes. Therefore, to produce healthy carrots it is much more critical to control root-knot nematodes prior to their growth than it would be prior to growing a crop such as yellow squash which will more readily tolerate feeding by these nematodes.
Control of plant-parasitic nematodes is best achieved by integrating two or more of the tactics provided above. It is very important to understand that to achieve control in any given environment it is imperative plant-parasitic nematodes are properly identified. Not all nematodes are the same.
The only way to detect and diagnose nematode problems and to assess their population densities is by collecting soil and/or plant tissue samples and having those samples processed in a nematology laboratory. It is highly recommended that if you believe you are experiencing a nematode problem, have it diagnosed and then consult with a nematologist in your state for management options.



 Print
Print Email
Email